Enteric Technology
Rethinking gastro-resistant enteric encapsulations
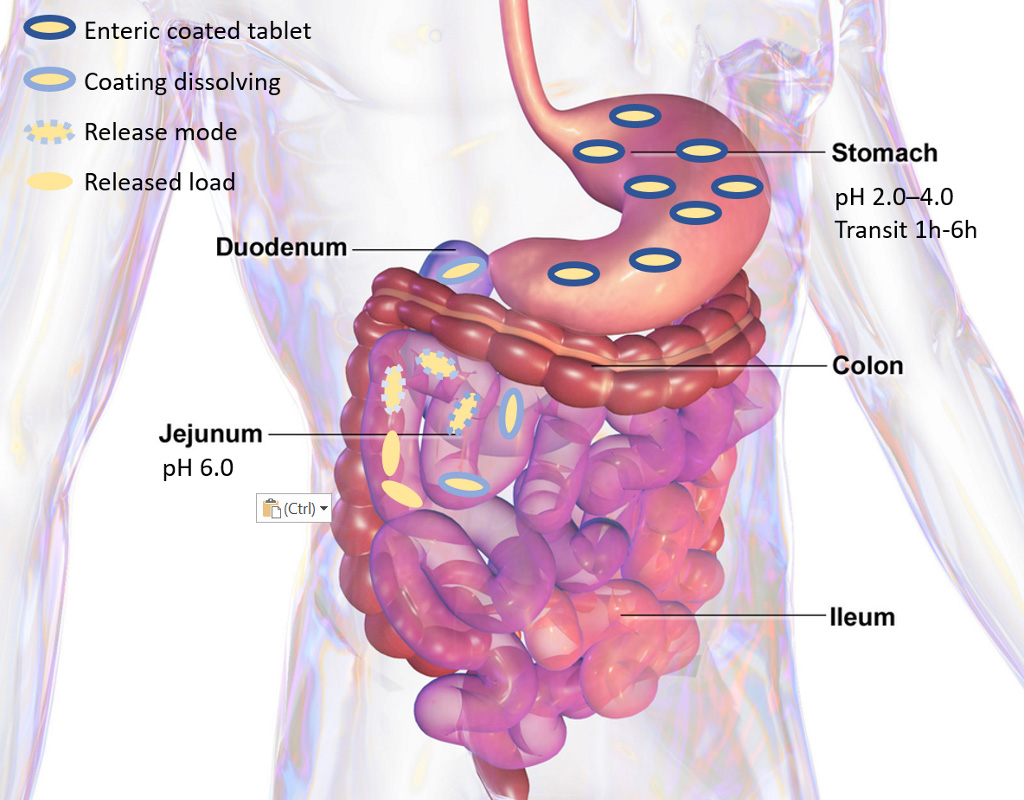
Gastro-resistant capsules with targeted release in the intestine must overcome highly variable stomach transition times.
Stomach transition times can vary from several minutes to many hours depending on individual fed state and physiological variability. Employing a time dependent mechanism for passing the stomach is therefore not an option for accurate intestinal delivery.
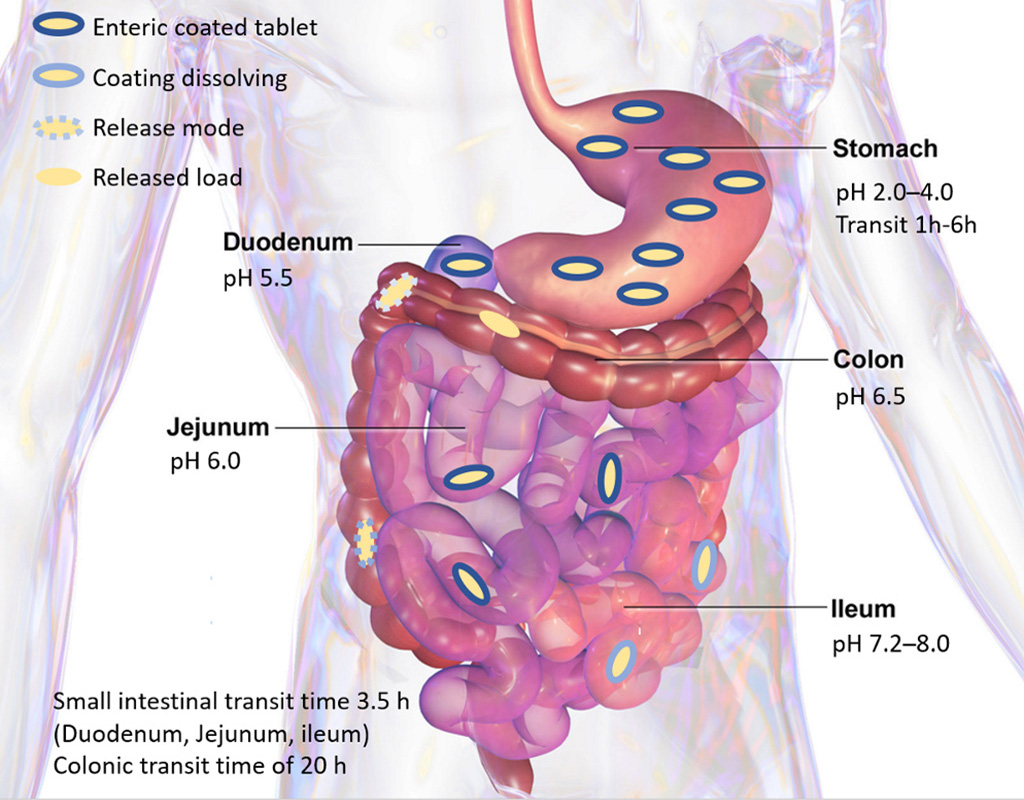
When the capsule reaches the intestine the transit time becomes relatively constant, and a time dependent release mechanism can therefore be employed. APIVault and NutraVault capsules are designed to release either rapidly for targeted delivery in the small intestine, or to dissolve slowly for targeted release in the colon. This gives new possibilities for intestinal targeting compared to the capsules already on the market.
The dissolution rate of the capsule is constant in the intestine. This, together with the constant intestinal transiting time, is used to deliver drugs or nutraceuticals anywhere in the intestine.

How do we use the pH difference?
APIVault and NutraVault capsules use the pH difference between the stomach and the intestine to govern release. The capsules are tight and stable under the acidic pH in the stomach, and dissolves at a controlled rate when reaching the neutral pH in the intestine.
Our capsule technology
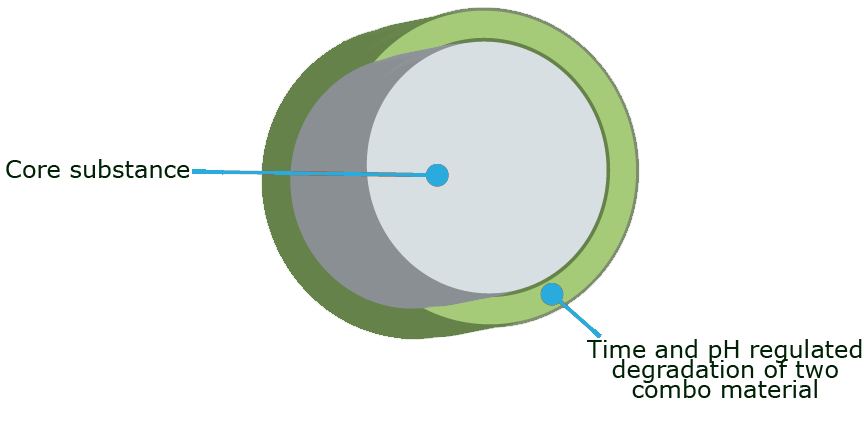
The capsule technology is built around a two-component encapsulation consisting of:
- Bacterial cellulose (BC). BC is a pure water insoluble form of cellulose. Cellulose fibers are not digested in the human GI tract as there are not naturally occurring cellulases.
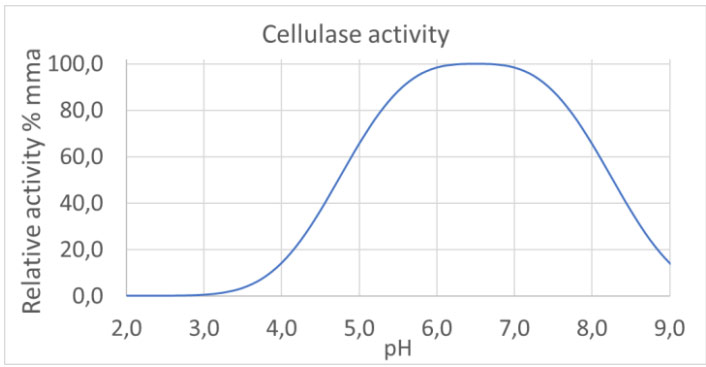
- pH dependent cellulases that are inhibited, and therefore inactive in the acidic stomach, but become active and digest BC in the pH neutral intestine
By infusing a well-defined cellulase into a specific BC, in a simple process, a dried two-component material is obtained with the cellulase contained in the BC membrane. The cellulase become active in the presence of water and at the right pH.
We have an in-depth understanding of the central control parameters in the process and targeted release in the intestine can therefore be achieved.

The potential of our technology
APIVault capsules are suitable for the development of a broad range of highly differentiated new pharmaceutical products.
NutraVault encapsulations are suitable for the development of a broad range of new nutraceuticals, prebiotics, probiotics, enzyme therapeutics and dietary Supplements.
In both cases this also includes products already marketed but formulated with a less efficient enteric coating technology.
The raw materials for the capsules are cellulose and cellulase. Cellulose is already well known in pharmaceutical production, where it has been used for many years for a variety of purposes. Cellulase is a main component in the production of biofuels and has been intensively studied for decades.
The production process for the encapsulation technology is a new combination of known bioprocess steps, and the cost per capsule is expected to be advantageous.
The advantages
The advantages of APIVault and NeutraVault being based on biocompatible non-toxic ingredients are several.
- With APIVault an easy regulatory approval of drugs utilizing the technology is expected and few restrictions to its use in humans.
- With NeutraVault cellulose and cellulases has been approved for dietary intake in some regions of the world, and approval is expected in major markets soon.
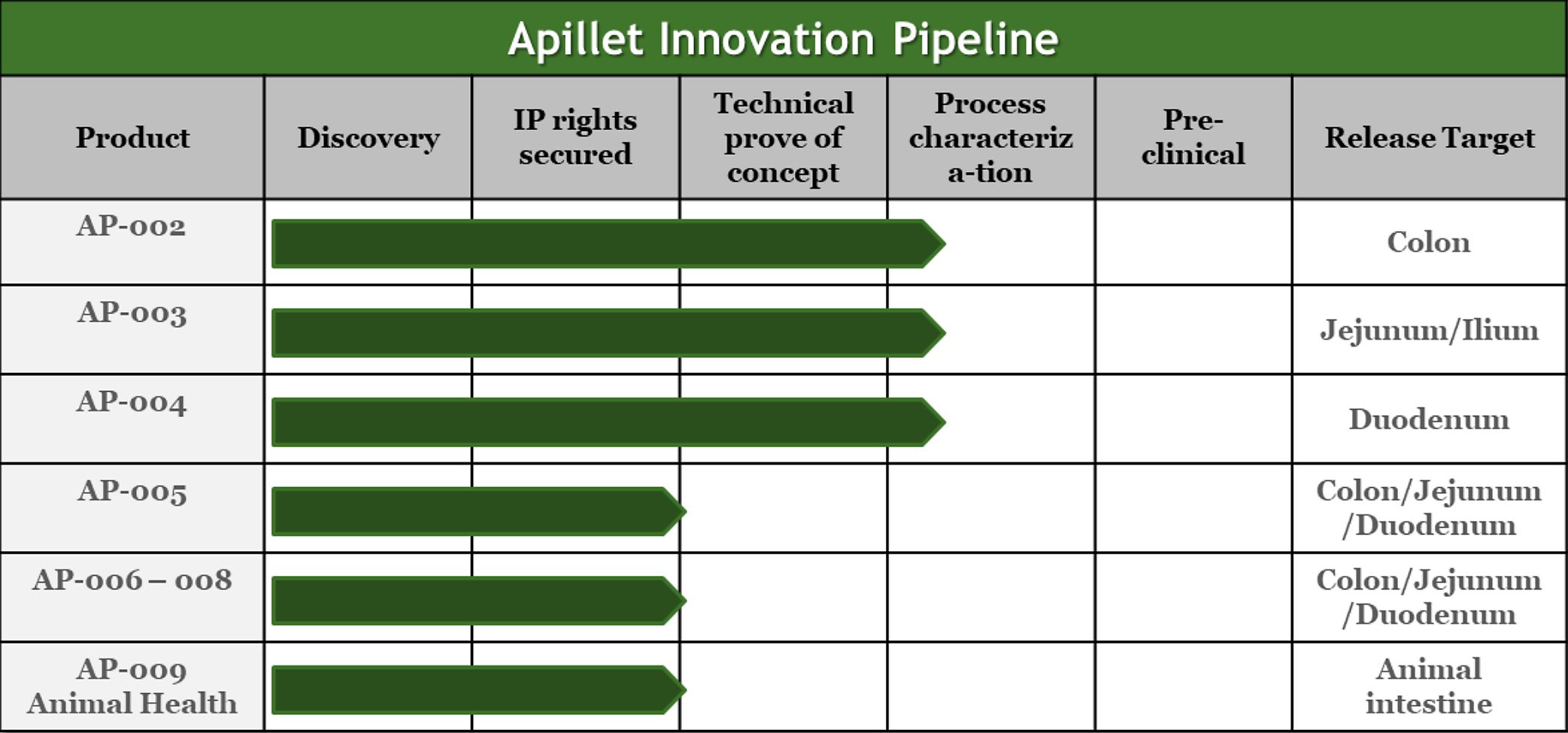
Apillet Innovation Pipeline
We are currently pursuing technical development of APIVault and NutraVault based on cellulose and cellulase. However, our patent covers encapsulations made of two-component coatings composed of dietary fibers and enzymes that digest dietary fibers. E.g., cellulose and cellulase (APIVault, NutraVault), pectin and pectinolytic enzymes, hemicellulose and hemicellulase, lignin and lignin degrading enzymes and fructan and fructan degrading enzymes.
There are therefore huge opportunities for new products with exciting new encapsulation features covered in the patent.
Intellectual Property Rights
A PCT application covering our novel technology resulted in a positive opinion from the international searching authority on all three accounts: the novelty, the inventive step, and the industrial applicability of the invention.
The invention has the priority date: August 14. 2018.
National patents on the pioneering encapsulation technology behind our APIVault and NutraVault lines of gastro-resistant enteric encapsulation are currently being pursued in all major markets.
As of October 4. 2023, a European Patent covers the encapsulation technology. The patent offers IP protection in the countries participating in the European Patent Convention:
Albania, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Monaco, Montenegro, Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, and the United Kingdom.